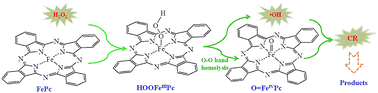
Leafy graphite nanosheet anchored iron(II) phthalocyanine nanorods (FePc@LGNS) were facilely synthesized without using a complex covalent anchoring procedure. FE-SEM, XRD, FTIR, and XPS characterizations confirmed the molecular configuration of FePc on the LGNS surface. The interlaced hydrophobic/hydrophilic regions and large specific-surface-area of the FePc@LGNS hybrid not only improved the adsorption capacity, but also promoted the oxidative ability of the FePc@LGNS–H2O2 system due to sufficient FePc catalytic active sites on LGNS surface. The optimal conditions for CR removal were initially pH 6.98, 50 mM H2O2 and 1.0 g L−1 FePc@LGNS hybrid. Different from the classical Fenton process, high-valent iron(IV)–oxo complexes and hydroxyl radicals are responsible for Congo red (CR) oxidative degradation. Liquid chromatography-mass spectrometry (LC-MS) analysis demonstrated the effective cleavage of both azo bonds and C–C bonds of CR molecules. A plausible oxidation mechanism of the FePc@LGNS–H2O2 system and the degradation pathway of CR were proposed. This FePc@LGNS–H2O2 system could be a highly efficient oxidation process for recalcitrant pollutants elimination over a wide pH range.