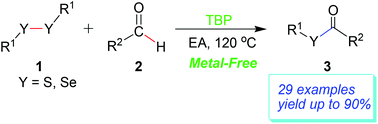
Thiol and selenol esters were synthesized by a direct oxidative coupling reaction of aldehydes with disulfides or diselenides in ethyl acetate under metal-free conditions. Among the oxidants examined, tert-butyl peroxide (TBP) was shown to give the best results. For the substrates with both electron-donating and electron-withdrawing substituents, the reaction proceeded smoothly and gave moderate to good yields. Compared with the previous method, the present route is very simple, atom-economical and environmentally friendly.