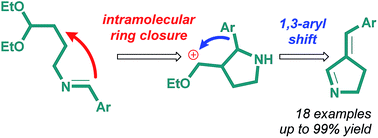
In this article, a novel tandem reaction, which transforms N-(4,4-diethoxybutyl)imines into 3-arylidene-1-pyrrolines, is described. The substrate scope of the starting acetals includes arenes with electron-donating and withdrawing groups. The X-ray study of products confirmed the E-stereochemistry of the double bonds formed. The best yields (99%) are found in boiling xylene in the presence of TsOH (or 2-nitroresocinol) during 40 (50) hours. The study of substituents effect on the course of the reaction revealed that cascade process takes place, combining acid-catalyzed intramolecular cyclization of N-(4,4-diethoxybutyl)imines and unusual 1,3-sigmatropic shift of the aryl fragment. Cyclic imines that are formed in high/excellent yields are of interest both from the viewpoint of their biological activity and synthetic usefulness.