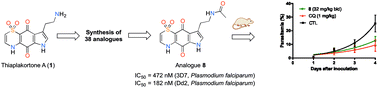
A series of amide (8–32, 40–45) and urea (33, 34, 36–39) analogues based on the thiaplakortone A natural product scaffold were synthesised and screened for in vitro antimalarial activity against chloroquine-sensitive (3D7) and chloroquine- and mefloquine-resistant (Dd2) Plasmodium falciparum parasite lines. Several analogues displayed potent inhibition of P. falciparum growth (IC50 <500 nM) and good selectivity for P. falciparum versus human neonatal foreskin fibroblast cells (selectivity index >100). Two of these compounds, 8 and 33, exhibited good aqueous solubility and metabolic stability, and when administered subcutaneously to mice (32 mg kg−1), plasma concentrations remained above 0.2 μM for at least 8 h. Both 8 and 33 were well tolerated in mice after subcutaneous administration of 32 mg kg−1 twice daily for 4 days. Using this regimen blood stage P. berghei was suppressed by 52% for 8 and 26% for 33, relative to the vehicle control.