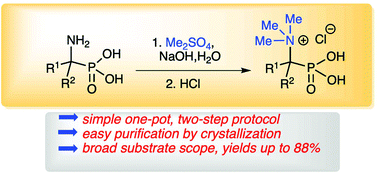
An effective protocol for the quaternization of simple 1-aminoalkylphosphonic acids under basic conditions using Me2SO4 as a convenient alkylating agent is reported. During the course of the reaction, phosphonic acid quaternary ammonium derivatives, along with their corresponding monoesters are formed. Subsequent direct acidic hydrolysis of the crude reaction mixture leads to the desired novel N,N,N-trialkyl-N-(1-phosphonoalkyl)ammonium salts with overall yields of up to 88%. The developed protocol is general in scope and the products are purified by simple crystallization to give stable solids. Novel quaternary ammonium salts bearing a phosphonic group are generally unreactive in acidic and alkaline media. However, some of them undergo Hofmann elimination and substitution reactions in the presence of a base.