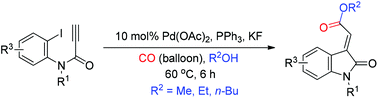Tandem reactions use consecutive reaction steps to efficiently synthesize compounds of high molecular complexity. This paper presents a tandem Pd-catalyzed Heck and alkoxycarbonylation reaction for the stereoselective synthesis of (E)-oxindolylidene acetates. The mechanism underlying the Pd-catalyzed tandem reaction involves the syn-carbopalladation of ynamides followed by alkoxycarbonylation with CO and alcohol. This method makes it possible to obtain the desired (E)-configuration of oxindolylidene acetates exclusively. We evaluated the scope of the reaction by applying optimal reaction conditions to the facile synthesis of a library of (E)-oxindolylidene acetates. The resulting (E)-oxindolylidene acetates exhibited potent anticancer activities against a variety of human cancer cell lines. The anticancer activities of some (E)-oxindolylidene acetates were even superior to those of known CDK inhibitors indirubin-3′-oxime and roscovitine.