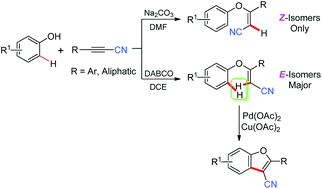
A Na2CO3-promoted addition of phenols to propiolonitriles generated (Z)-3-aryloxy-acrylonitriles in nearly quantitative yields with exclusively Z-isomers, and a DABCO-promoted addition reaction of phenols with propiolonitriles afforded mainly (E)-3-aryloxy-acrylonitriles with high yields. The obtained (E)-3-aryloxy-acrylonitriles underwent intramolecular cyclization to give 3-cyanobenzofurans in good yields through palladium-catalyzed direct C–H bond functionalization.