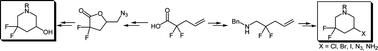
Synthetic routes toward new 5-amino- and 5-hydroxy-3,3-difluoropiperidines, which are of high interest as building blocks in medicinal chemistry, are described. The key step involves the N-halosuccinimide-induced cyclization of 2,2-difluoro-4-pentenylamines toward 5-halo-3,3-difluoropiperidines, which were used to synthesize 5-amino-3,3-difluoropiperidine. In a second strategy, iodolactonization of 2,2-difluoro-4-pentenoic acid gave the corresponding γ-lactone, which was transformed into 5-hydroxy-3,3-difluoropiperidine.