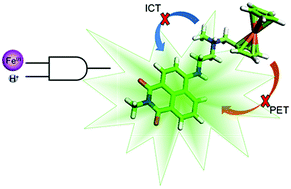
Two novel naphthalimide-based ‘Pourbaix sensors’ for redox potential and pH were designed based on a ‘fluorophore–spacer1–receptor–spacer2–electron-donor’ configuration. The synthesised molecular logic gates consist of an alkylated 1,8-naphthalimide fluorophore connected to a tertiary amine by a flexible ethylene spacer to a ferrocene moiety via a methylene spacer. The UV-visible absorption and steady state fluorescent properties were examined in methanol and 1 : 1 (v/v) methanol/water. The spectroscopic properties are modulated by internal charge transfer (ICT) and photoinduced electron transfer (PET) mechanisms. A log βH+ of 9.2 and 8.7 were determined in 1 : 1 (v/v) methanol/water for the methylated 1 and butylated 2 compounds, respectively. An apparent log βFe3+ of 4.2 was determined in 1 : 1 (v/v) methanol/water at pH 4. Time-resolved spectroscopic studies elucidated the stimulus-modulated photoinduced electron transfer pathways. In the oxidised and protonated state, 1 exhibits a single fluorescence lifetime of 8.5 ns, while an efficient photoinduced electron transfer characterised by a time constant of 20 ps is revealed by femtosecond transient absorption spectroscopy in the absence of a perturbing stimulus.