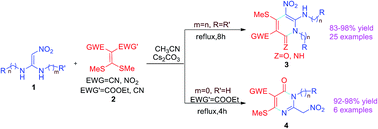
The methods for the synthesis of two novel types of compounds, including pyridin-2-ones 3 and pyrimidin-4-ones 4 were developed. Pyridin-2-ones 3 were synthesised via the regioselective reaction of N,N′-disubstituted 1,1-ene diamines 1a–1w with mercaptals 2a–2c in acetonitrile promoted by Cs2CO3 under refluxing conditions. Fortunately, pyrimidin-4-ones 4 were obtained when the N-monosubstituted 1,1-ene diamines 1x–1b′, used as substrate, by accident, reacted with mercaptals 2 under similar conditions. As a result, two kinds of novel heterocycles were synthesised by this protocol. The reactions have some advantages, such as excellent yield, inexpensive raw materials and convenient final treatment. The antitumor bioactivity screening showed that certain compounds had potent antitumor activity. Especially, compounds 3r, which showed the most potent activity with IC50 values lower than 12.3 μmol L−1 against four human tumor cell lines, making it more active than cisplatin (DDP). In addition, a preliminary assessment of the structure–selectivity relationship of the compounds was also performed.