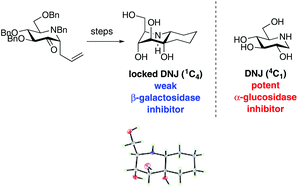
A series of analogs of the iminosugars 1-deoxynojirimycin (DNJ) and 1-deoxymannojirimycin (DMJ), in which an extra five or six-membered ring has been fused to the C1–C2 bond have been prepared. The synthetic strategy exploits a key 2-keto-C-allyl iminosugar, easily accessible from gluconolactam, which upon Grignard addition and RCM furnishes a bicyclic scaffold that can be further hydroxylated at the C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C bond. This strategy furnished DNJ mimics with the piperidine ring locked in a 1C4 conformation with all substituents in axial orientation when fused to a six-membered ring. Addition of an extra ring to DNJ and DMJ motif proved to strongly modify the glycosidase inhibition profile of the parent iminosugars leading to modest inhibitors. The 2-keto-C-allyl iminosugar scaffold was further used to access N-acetylglycosamine analogs via oxime formation.
C bond. This strategy furnished DNJ mimics with the piperidine ring locked in a 1C4 conformation with all substituents in axial orientation when fused to a six-membered ring. Addition of an extra ring to DNJ and DMJ motif proved to strongly modify the glycosidase inhibition profile of the parent iminosugars leading to modest inhibitors. The 2-keto-C-allyl iminosugar scaffold was further used to access N-acetylglycosamine analogs via oxime formation.