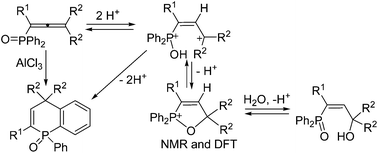
1-(Diphenylphosphoryl)alka-1,2-dienes (phosphonoallenes) in Brønsted (super)acids (TfOH, FSO3H, H2SO4) gave the corresponding 1,2-oxaphosphol-3-enium ions, that were studied by means of NMR and DFT calculations. Upon hydrolysis of reaction solution, these cations afforded 3-hydroxyalk-2-en-1-yl-diphenylphosphine oxides (phosphonoallyl alcohols). But in (super)acids the cations were slowly transformed into O-protonated forms of 1-phenyl-1,4-dihydrophosphinoline 1-oxides, which were monitored by NMR. The latter phosphaheterocycles can be directly obtained from phosphonoallenes under the action of Lewis acid AlCl3.