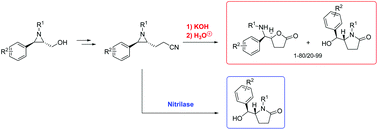
Trans- and cis-2-aryl-3-(2-cyanoethyl)aziridines, prepared via alkylation of the corresponding 2-aryl-3-(tosyloxymethyl)aziridines with the sodium salt of trimethylsilylacetonitrile, were transformed into variable mixtures of 4-[aryl(alkylamino)methyl]butyrolactones and 5-[aryl(hydroxy)methyl]pyrrolidin-2-ones via KOH-mediated hydrolysis of the cyano group, followed by ring expansion. In addition, next to this chemical approach, enzymatic hydrolysis of the former aziridinyl nitriles by means of a nitrilase was performed as well, interestingly providing a selective route towards the above-mentioned functionalized γ-lactams.