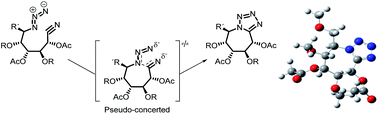
The synthesis of novel tetrazolo azepanes from azido nitriles by 1,3 intramolecular dipolar cycloaddition starting from monosaccharide derivatives is described. A quantum chemical topological study on the intramolecular cyclization process has been conducted rendering a pseudo-concerted mechanism. Conformational study was done for the final products which showed a preferential twist boat conformation, theoretically suitable for mannosidase inhibition. However, the tetrazoles showed no significant inhibition of glycosidases.