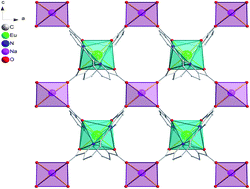
Solvothermal reactions of Ln(NO3)3·6H2O and 2,2′-bipyridine-6,6′-dicarboxylic acid (H2bpyda) in MeOH in the presence of alkali metal (Na or K) hydrate lead to four novel alkali–lanthanide heterometallic coordination polymers: [LnNa(bpyda)2]n (Ln = Eu 1, Tb 2) and [LnK(bpyda)2(MeOH)]n (Ln = Eu 3, Tb 4). 1 and 2 are isostructural and crystallize in the orthorhombic Pccn space group and the isostructural 3 and 4 are in the monoclinic P21/c space group. In all compounds, both [Ln(bpyda)2]− subunits and the alkali metals (Na+/K+) act as four-connectors to form the 2D (4,4) layer structures. Luminescent measurements in both solid-state (for 1 and 2) and MeOH solution (for 1–4) reveal that all compounds exhibit the characteristic emission bands of the lanthanide in the visible region and the strongest emissions are 5D0 → 7F2 for 1 and 3, and 5D4 → 7F5 transitions for 2 and 4.