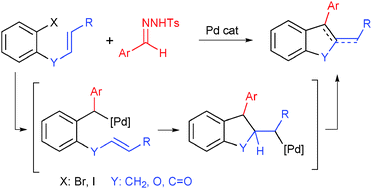
The Pd-catalyzed reaction between o-iodoallylbenzene and tosylhydrazones gives rise to indene derivatives through a process that involves a carbene migratory insertion followed by an intramolecular carbopalladation, with the formation of two C–C bonds on the same carbon atom. The same strategy has also been applied for the synthesis of 2,3-disubstituted benzofurans and indenones by selecting the appropriate o-substituted iodoarene.