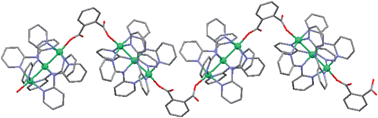
The reaction between [Ni3(dpa)4(ClO4)2] and phenyldicarboxylates led to three coordination compounds based on trinickel clusters [Ni3(dap)4]2+, [Ni3(dpa)4(1,2-Hbdc)2]·CH2Cl2·H2O (1), {[Ni3(dpa)4(1,2-bdc)]·1.5H2O}n (2), and {[Ni3(dpa)4(1,2-nbdc)]·1.5H2O}n (3) where dpa− is 2,2′-dipyridylamine anion, 1,2-H2bdc is 1,2-benzenedicarboxylic acid, and 1,2-H2nbdc is 3-nitro-1,2-benzenedicarboxylic acid. Complexes 2 and 3 were obtained in the case of introducing of water, which is completely different from synthetic methods for previously reported complexes with trinickel clusters [Ni3(dap)4]2+. Complex 1 is a trinickel string complex with two 1,2-Hbdc− axial ligands, while complexes 2 and 3 are 1D polymers with bridged phenyldicarboxylates. Axial replacement of phenyldicarboxylates results in some structural changes in the trinickel clusters and some changes of properties, such as solubility, electrochemical behaviour, and fluorescence emission. Desolvated 1 is less stable than those of complexes 2 and 3, indicating the polymeric assemblies enhance the packing stability. The π–π interactions occur in complexes 1–3 and the weakest interaction in complex 2. Moreover, complexes 2 and 3 have 1D ladder hydrogen-bonding chains.