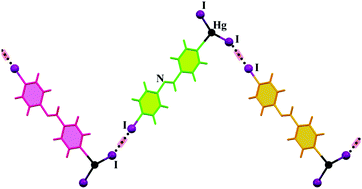
A series of eight new Hg(II) complexes based on the L4-X ligands, where L is (E) -4-halo-N-(pyridin-4-ylmethylene)aniline, were synthesized and characterized and their supramolecular crystal structures were studied by different geometrical and theoretical methods. Our study reveals the role of weak intermolecular interactions involving halogens, such as C–H⋯X hydrogen bonds (in the cases of 1, 2, 3, 5, 6 and 7) and C–X⋯X′–M halogen bonds (in the cases of 4 and 8), in the structural changes of supramolecular assemblies of coordination compounds. Complexes 1–8 were also synthesized by sonochemical irradiation and the morphology of the prepared complexes was investigated using FE-SEM. The BFDH analysis helps us to compare the predicted morphology to that obtained under ultrasonication. This study may provide further insight into discovering the role of weak intermolecular interactions in the context of metallosupramolecular assembly.