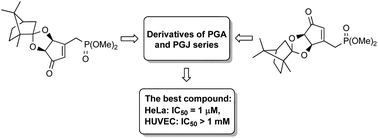
The synthesis of two cross-conjugated prostaglandin analogues of known neurotrophic activity and their new hydroxy derivatives was accomplished starting from the diastereoisomeric (+)-camphor protected 3-[(dimethoxyphosphoryl)methyl]-4,5-dihydroxycyclopent-2-enones. The cytotoxicity of these compounds was determined against HeLa, K562, HL-60 human cancer cell lines and normal human cells (HUVEC). We found that NEPP11 and its C7-hydroxy derivative demonstrated high anticancer activity against the HeLa and HL-60 human cancer cell lines at concentrations ranging from 1 to 2 μM. Moreover, the C7-hydroxy derivative of NEPP11 displayed high cytotoxic selectivity between cancer cell lines and normal human cells. On the other hand, the J-type prostaglandin analogue of NEPP11 and its C13-hydroxy derivatives were much less toxic or nontoxic against the cancer and normal cells at concentrations up to 1 mM.