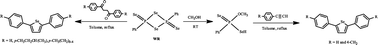
Reaction of 2,4-bis(phenyl)-1,3-diselenadiphosphetane-2,4-diselenide [PhP(Se)(μ-Se)]2 (Woollins’ reagent, WR) with one equivalent of 1,4-diarylbutane-1,4-diones 1a–g in refluxing toluene affords the corresponding 2,5-diarylselenophenes 2a–g in excellent yields (up to 99%). Alternatively, the 2,5-diarylselenophenes (2a and 2b) can be obtained in 70–80% yields from the reaction of arylacetylene with an equivalent of O-methyl Se-hydrogen phenylphosphonodiselenoate; the latter was derived from WR and methanol. The first X-ray structure of 2,5-diarylselenophenes is presented along with characterisation of their redox properties.