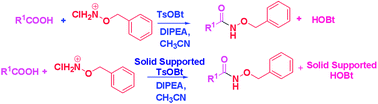
The direct conversion of various carboxylic acids, that include sterically hindered amino acids and di-peptides, to O-benzyl hydroxamates is demonstrated using sulfonate esters of benzotriazoles under ambient and milder conditions without significant racemization. This simple and efficient protocol is extended to the synthesis of O-benzyl hydroxamates, using in situ generated solid supported TsOBt to facilitate the recovery and re-usability of HOBt and to render the isolation of the products easier. Such in situ generation and further application of a coupling reagent is novel and industrially important.