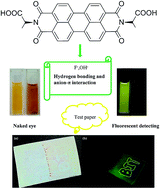
Four types of perylene diimide–amino acid derivatives (H2PDIAla, H2PDIGlu, H2PDIPhe, H2PDITyr) were chosen to interact with anions. The UV-vis, fluorescence (in DMF solution) and NMR spectra in DMSO-d6 solution were studied. The results indicated that the four types of derivatives exhibit specific selectivity and high sensitivity for F− and OH− ions. The NMR titrations of the derivatives with F− and OH− ions confirmed the synergetic effect of hydrogen bonding and anion–π interaction between the derivatives and the anions (F−, OH−). In addition, we made a simple test-paper to develop its potential applications.