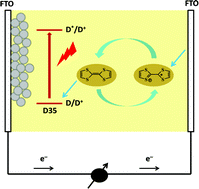
Tetrathiafulvalene (TTF) was investigated as an organic iodine-free redox mediator in electrolytes for dye-sensitized, nanocrystalline solar cells (DSCs) and was compared to the commonly used iodide/triiodide system. The TTF system studied was determined to be a one-electron transfer system, although potentially exhibiting three well-defined oxidation states. Despite the slightly positive redox potential of TTF, electrolytes with TTF displayed around 200 mV lower open-circuit voltage than the iodide/triiodide system. This can mainly be ascribed to a much shorter electron lifetime in the TiO2 film. Mass transport limitations for redox species in TTF-based electrolytes were found to be serious. Electrochemical impedance measurements (EIS) show that the charge-transfer resistance at the counter electrode in the electrolyte with TTF is considerably larger than for the iodide/triiodide system. In addition, the light absorption of the TTF-based electrolyte is stronger than that for the iodide/triiodide system. Thus, DSCs with TTF-based electrolytes show worse photovoltaic performance than those with iodide/triiodide-based electrolytes. The differences in I–V characteristics and charge-recombination behavior have also been elucidated.