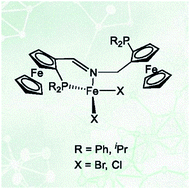
Five new chiral PNP ferrocene ligands with either an imine or amine nitrogen coordination site were synthesized. Only the imine type ligands formed Fe(II) complexes with the general formula [Fe(PNP)X2] (X = Cl, Br). In the solid state these complexes adopt a tetrahedral geometry with the PNP ligand coordinated in a κ2P,N-fashion with the one pendant-arm and the other not coordinated, as determined by X-ray crystallography and Mössbauer spectroscopy. The complexes are paramagnetic with a quintet ground state. In solution there is an equilibrium between [Fe(κ3P,N,P-PNP)X2] and [Fe(κ2P,N-PNP)X2] complexes. Boronation of the non-coordinated arm shifts the equilibrium towards the four-coordinate complex [Fe(κ2P,N-PNPBH3)Br2]. DFT calculations are consistent with the experimental results and indicate that the experimentally observed κ2 isomer is thermodynamically the most stable. In a CO atmosphere, [Fe(PNP)(CO)2Br]Br was formed rather than [Fe(PNP)(CO)Br2].