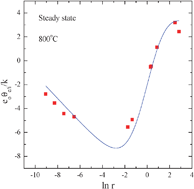
The thermoelectric power of a mixed ionic electronic conductor oxide Ce1−xGdxO2−x/2−δ (x = 0.1) was measured as a function of oxygen activity in the range of −20 ≤ log aO2≤ 0 at different temperatures in the range of 800–1100 °C, supposedly in two different states of the oxide with respect to the oxygen nonstoichiometry (δ) distribution: ∇δ = 0 and ∇δ≠ 0. The two sets of thermopower, corresponding to these two different nonstoichiometry distributions, generally change their sign from negative to positive, as the electrical conductivity changes from ionic to electronic with decreasing oxygen partial pressure from 1 atm, indicating a change in carrier types from positive to negative carriers. The two, however, exhibit a significant difference in magnitude (normally up to 400 μV K−1) particularly in the transition region from ionic to electronic conduction. The thermopower in the two different states has been described thermodynamically as a function of oxygen partial pressure or the electronic to ionic conductivity ratio. It has been found that the kinetic term An of the electronic thermopower [as in θn = (k/e)(ln Nc/n + An)] takes a numerical value 2 as the electronic transference number approaches 1, quite contrary to the theoretically predicted value 0 for a small polaron conduction mechanism.