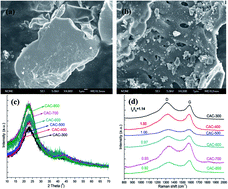
Biomass activated carbons were activated and pyrolyzed at 300–800 °C under nitrogen circumstance (named as CAC300–CAC800). The physicochemical characteristics of CACs and mechanisms of perchlorate (ClO4−) adsorption on CACs were investigated. Compared to CAC300, CAC600–CAC800 had higher surface area, total prove volume, higher hydrophobic surface and an abundant of oxygen-containing groups (–COH and –COOH) which contributed to a higher ClO4− adsorption capacity. The pH value of solution significantly affected ClO4− adsorption and the maximum ClO4− adsorption capacity occurred at pH 2.0 (less than pHIEP), where surface charge was positive. Electrostatic attraction and hydrogen bonding to oxygen-containing groups on CACs were considered as the dominant force for ClO4− adsorption. With the pH increased, electrostatic attraction disappeared and surface oxygen-containing groups were gradually deprotonated, and the ClO4− adsorption amount was gradually decreased few until pH 12.0. The batch adsorption experiments of ClO4− showed that CAC600–CAC800 were effective adsorbents for the adsorption of ClO4−.