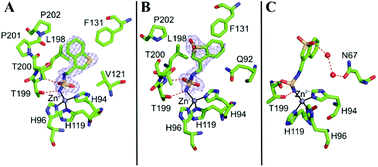
JNJ-26990990 ((benzo[b]thien-3-yl)methyl)sulfamide, a sulfamide derivative structurally related to the antiepileptic drug zonisamide, was reported to be devoid of carbonic anhydrase (CA, EC 4.2.1.1) inhibitory properties. Here we report that JNJ-26990990 and its S,S-dioxide analog significantly inhibit six human (h) isoforms, hCA I, II, VII, IX, XII and XIV, involved in crucial physiological processes. Inhibition and X-ray crystallographic data for the binding of the two compounds to these enzymes show significant similarity with the zonisamide inhibitory pattern. These findings prompted us to reconsider the structural/pharmacological requirements for designing effective antiepileptics possessing zinc-binding groups of the sulfamide, sulfamate or sulfonamide type in their molecules.