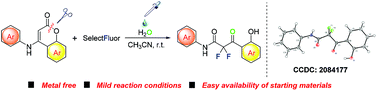
An expeditious approach to synthesize difluorinated 3-oxo-N,3-diarylpropanamides from 4-arylamino coumarins has been accomplished in the presence of Selectfluor, which plays the dual role of a mild oxidant and a source of fluorine. The use of five equivalents of water as an additive plays a key role in this tandem reaction, and the reaction will not occur in the absence of water. The reaction is proposed to involve the difluorination of 4-arylamino coumarins by Selectfluor via a radical process, cleavage of the in situ generated imines, and an amide-formation reaction. The products are confirmed unambiguously from their spectra and by single-crystal X-ray analysis, and are found to exhibit certain hypoglycemic activity.