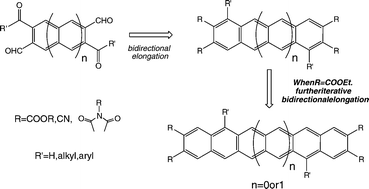
Previously, we developed an iterative elongation methodology to synthesize acene esters, nitriles, and imides. The strategy uses the concept of bidirectional synthesis, and we can now make a series of electron deficient anthracene, tetracene, and pentacene derivatives via the bidirectional iterative elongation protocol. Central units, used to initiate the bidirectional elongation, were synthesized by employing a double anionic Fries rearrangement as the key step. The photophysical and electrochemical properties of these novel electron acceptors are investigated and interpreted based on the electron withdrawing power of the substitutions. An excited state charge transfer was proposed for one compound to account for its peculiar fluorescent behavior.