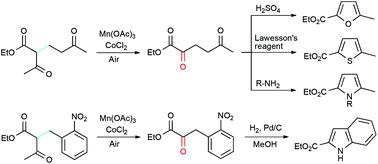
One pot syntheses of furan, thiophene, and pyrrole were accomplished by oxidative deacetylation using Mn(III)/Co(II) catalysts and the Paal–Knorr reaction from 1,5-dicarbonyl compounds, which are prepared from the conjugate addition of ethyl acetoacetate to α,β-unsaturated carbonyl compounds. The oxidative deacetylation and reductive cyclization of β-ketoesters derived from ethyl acetoacetate and o-nitrobenzyl bromides efficiently produced diversely substituted indoles.