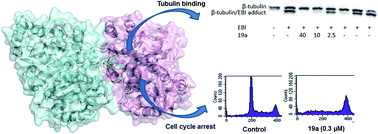
Cyclohexanedione derivatives represent a new family of colchicine-site binders that were identified through a ligand-based virtual screening approach. Structural modifications have now been performed at both distal sites of our identified hit [2-(1-((2-methoxyphenyl)amino)ethylidene)-5-phenylcyclohexane-1,3-dione (4)] in order to improve tubulin binding affinity, anti-proliferative activity and/or aqueous solubility. The results obtained indicate that the 2-methoxyphenyl ring, the fragment located closer to the αβ-tubulin interface according to docking studies, is the one that allows structural variation in order to improve the Ka value against tubulin (as in compound 20a with a Ka = 1.3 × 107 M−1, analogous to colchicine) or to improve aqueous solubility, as in compound 22c, being more than 10 times more soluble than the previous hit 4.