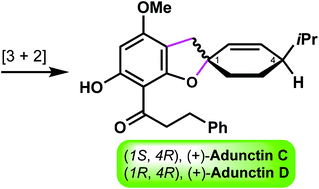
The first total synthesis of (+)-adunctin C (ent-1) and (+)-adunctin D (2), two monoterpene-substitued dihydrochalcones isolated from Piper aduncum (Piperaceae), was achieved. A regioselective oxidative [3 + 2] cycloaddition of acylphloroglucinol with (−)-β-phellandrene was developed to construct their unique spirobenzofuran skeleton. The absolute configurations of natural adunctins 1 and 2 were thus assigned through these endeavors.