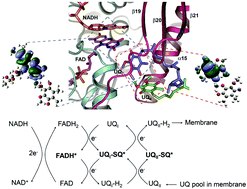
Ndi1 is a special type-II complex I nicotinamide-adenine-dinucleotide (NADH):ubiquinone (UQ) oxidoreductase in the yeast respiratory chain, with two bound UQs (UQI and UQII) mediating electron transfer from flavin cofactors to ubiquinone, in the absence of Fe–S chains. Here, we elucidate the underlying mechanism of electron transfer in Ndi1 through temperature-dependent Electron Spin Resonance (ESR) experiments in conjunction with quantum chemical calculations. It is revealed that electron transfer is mediated by antiferromagnetic (AFM) interactions between flavin-adenosine-dinucleotide (FAD) and UQI and between UQI and UQII. The π-stacking interactions among the aromatic complexes also enhance the through-space electron transfer. The FAD/UQI pair works as a rectifier converting double-electron co-transfer into sequential single-electron transfer events. The results not only expand our understanding on the observed AFM interactions among p-orbital aromatic mixed-stack in proteins, but also provide significant insights into the fabrication of materials with special magnetic properties using biological samples.