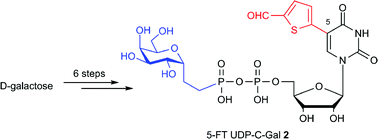
Structural analogues and mimics of the natural sugar-nucleotide UDP-galactose (UDP-Gal) are sought after as chemical tools for glycobiology and drug discovery. We have recently developed a novel class of galactosyltransferase (GalT) inhibitors derived from UDP-Gal, bearing an additional substituent at the 5-position of the uracil base. Herein we report the first C-glycosidic derivative of this new class of GalT inhibitors. We describe a practical convergent synthesis of the new UDP-C-Gal derivative, including a systematic study into the use of radical chemistry for the preparation of galactosyl ethylphosphonate, a key synthetic intermediate. The new inhibitor showed activity against a bacterial UDP-Gal 4′-epimerase at micromolar concentrations. This is the first example of a base-modified UDP-sugar as an inhibitor of a UDP-sugar-dependent enzyme which is not a glycosyltransferase, and these results may therefore have implications for the design of inhibitors of these enzymes in the future.