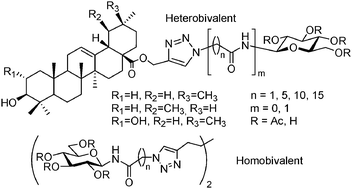
Propargyl esters of the C-28 carboxylic acids of pentacyclic triterpenes (oleanolic, ursolic, and maslinic acids) were coupled with 2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl azide as well as N-(ω-azido-[C-2, C-6, and C-11]alkanoyl)-β-D-glucopyranosylamines under conditions of copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) to give tethered D-glucose–triterpene heteroconjugates. The O-acetyl protecting groups were removed by base-catalyzed hydrolysis. N-(ω-Azido-[C-2, C-6, C-11, and C-16]alkanoyl)-β-D-glucopyranosylamines were also tethered by 1,7-octadiyne under CuAAC conditions to furnish D-glucose homoconjugates. O-Deacetylation was carried out by the Zemplén protocol. The new compounds were assayed against rabbit muscle glycogen phosphorylase (RMGP) a or b enzymes. Some of the heteroconjugates inhibited the enzyme in the low micromolar range (IC50 values 40–70 μM), while the homoconjugates proved inefficient as inhibitors.