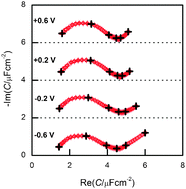
Five hexaalkylguanidinium-based ionic liquids have been synthesised, and based on their cyclic voltammograms the most suited one, N,N-dibutyl-N′,N′-diethyl-N′′,N′′-dimethylguanidinium bis(trifluoromethylsulfonyl)imide, has been chosen for electrochemical studies. The surface interaction of this room-temperature ionic liquid with single crystalline gold surfaces (Au(100) and Au(111)) has been investigated using cyclic voltammetry, impedance spectroscopy and in situ scanning tunnelling microscopy (STM). The interfacial capacitance was found to be very low; STM measurements revealed the hex-reconstruction and herringbone reconstruction for Au(100) and for Au(111), respectively, at negative potentials; that is, at these potentials no hints for ad-structures of the cation could be found.