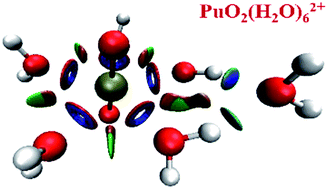
The equilibrium structure, stabilities, electronic structures, chemical bonding and topological properties of PuO2(H2O)n2+ (n = 1–6) complexes in the gas phase have been systematically investigated by different levels of theory. The results indicate that all the ground states of these complexes are triplet. The five water molecules of PuO2(H2O)m2+ (m = 1–5) are arranged on the equatorial plane of plutonyl. Reactivity analysis of PuO2(H2O)52+ shows that the oxygen atom of the sixth water molecule is connected by hydrogen-bonds to the two water molecules which are on the equatorial plane of PuO2(H2O)52+. The optimized geometries are in agreement with available theoretical and experimental results. The weak covalent interactions of Pu–ligand bonds were evaluated by the electron localization function and atoms in molecules analyses. The orbital interactions were investigated by analysis of total, partial, and overlap population density of state diagrams. Besides, a reduced density gradient approach was implemented to analyze the weak interactions and steric repulsions present in PuO2(H2O)52+ and PuO2(H2O)62+ complexes.