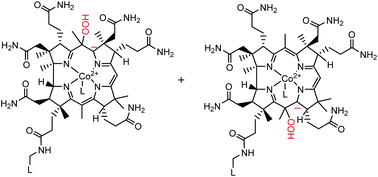
Comparative studies on the reactions of reduced cobalamin (Cbl(II)), the complexes of reduced cobalamin with the SO2−˙ radical anion (B12rs, SO2−Cbl(II)), and super reduced cobalamin (B12s, Cbl(I)) with hydrogen peroxide (H2O2) and tert-butyl hydroperoxide (tBuOOH) were carried out in aqueous solution under anaerobic conditions. Analyses of the reaction products indicated that cob(III)alamin (Cbl(III)) and meso-hydroxylated derivatives of vitamin B12 (stable yellow corrinoids, SYCs) are the main colored products of the reactions of H2O2 with Cbl(II) and SO2−Cbl(II). In contrast to H2O2, Cbl(III) is the only colored product of the reactions of reduced cobalamins with tBuOOH. Both peroxides react with super-reduced cobalamin to form Cbl(II) at the [(Cbl(I))] : [peroxides] = 2 : 1 ratio. Based on a comparison of possible reaction pathways for the formation of Cbl(II) oxidation products, we concluded that the Cbl(II) carbanions are the intermediates of the reaction of hydrogen peroxide with Cbl(II) in aqueous solution. Experiments with reduced cobinamides showed explicitly that the ribonucleotide tail of cobalamin does not affect the yield of SYCs.