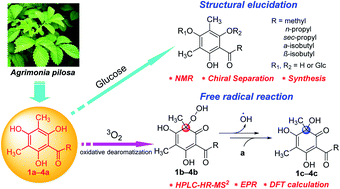
Six methyl-substituted phloroglucinol glycosides (1–6) were isolated from Agrimonia pilosa, including four new compounds (1–3, 6). The aglycones (1a–4a) of 1–4 and their corresponding oxidized products (1c–4c) were also obtained from A. pilosa. The structures were determined by a series of spectroscopic analyses and chiral separation. Notably, the structures of aglycones 1a–4a were unstable and prone to oxidation spontaneously, to yield the dearomatized structures 1c–4c. The mechanism of oxidative dearomatization was disclosed as a free-radical chain reaction with 3O2 by the techniques of HPLC-HR-MS2, EPR spectra and DFT-calculation, and hydroperoxide was defined as the intermediate.