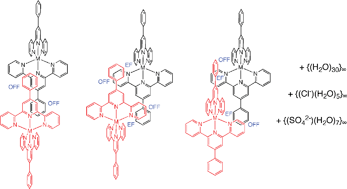
Previously we reported that complexes [M(Phterpy)2]2+ (Phterpy = 4′-phenyl-2,2′:6′,2″-terpyridine) when crystallised with PF6− generally form the standard two-dimensional terpy embrace (2D-TE) grid, except in one crystal where the 4′-phenyl substituents intercalate the 2D-TE, partially decomposing it to one-dimensional terpy embraces (1D-TE). Here we report that when crystallised instead with the hydrophilic anions Cl−, SO42−, tosylate, and [Ni(dipic)2]2− (dipic = pyridine-2,6-dicarboxylate) these complexes form a different class of layered structure. One layer contains the [M(Phterpy)2]2+ complexes, while in the other layer the anions are associated with aggregates of large numbers of water molecules and in some cases solvent alcohol molecules. Further, the [M(Phterpy)2]2+ complexes generally form longitudinal embraces, propagated in a direction parallel to the pseudo-S4 axes of the complexes, in contrast to the lateral embraces of the 2D-TE and 1D-TE motifs. The total numbers of offset-face-to-face and edge-to-face local interactions are similar for longitudinal and lateral embrace motifs. The crystals described are [Ru(Phterpy)2]Cl2(H2O)101, [Ru(Phterpy)2]Cl2(MeOH)4(H2O) 2, [Fe(Phterpy)2]SO4(iBuOH)0.5(H2O)7.53, [Ru(Phterpy)2][Ni(dipic)2](H2O)154, and [Ru(Phterpy)2](tosylate)2(H2O)45. Features of the hydrophilic domains in these crystals are sheets of {(Cl−)(H2O)5}∞ in 1, a chain of {(SO42−)(H2O)7}∞ in 3, and chains of beads (H2O)30 in 4. We conclude that the crystal engineering of extended embrace motifs for hydrophobic [M(Phterpy)2]2+ complexes is anion-dominated, through the degree of anion conscription of hydrogen-bonding solvent during crystallisation.