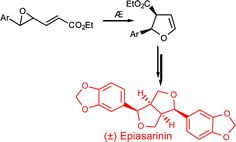
Flash vacuum pyrrolysis of vinyl epoxides provides cis-dihydrofuran carboxylic esters in good yields and diastereoselectivities, which, on base-promoted epimerisation afford the complementary trans series. The compounds provide a viable template for a Lewis acid promoted cyclisation to provide the 2,6-diaryl-3,7-dioxabicyclo[3.3.0]octane core found in the furofuran series of natural lignans. This strategy is stereodivergent and can be controlled to provide the exo-exo, exo-endo or endo-endo stereochemistries. The approach has been exemplified in syntheses of the sesamyl furofurans (±)-epiasarinin and (±)-asarinin.