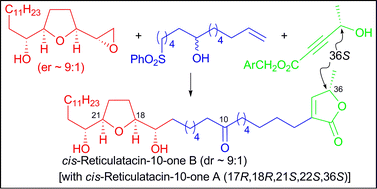
cis-Reticulatacin-10-ones A and B were synthesised as a predefined mixture of diastereoisomers (dr ∼ 1 : 9) in nine steps from the acid chloride 8, and without the use of hydroxyl protecting groups. Comparison of the chiral HPLC chromatogram of the synthetic sample with that of the natural product isolated from the roots of the tropical fruit tree Annona muricata L. showed the natural product to be a mixture of A and B diastereoisomers (dr ∼ 1 : 1).