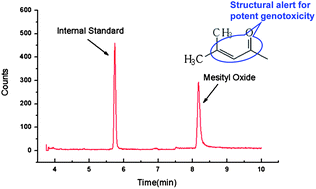
The determination of genotoxic impurities in drug substances requires analytical methods with high specificity and sensitivity. Mesityl oxide is a potent genotoxic impurity in drugs because its alpha-unsaturated ketone structure is considered to be a structural alert. A gas chromatograph electron ionization mass spectrometric (GC-EI-MS) method has been established for the determination of mesityl oxide in enalapril maleate in this article. Methyl isobutyl ketone was used as an internal standard (IS). The method was operated in electron ionization/selective ion monitoring (EI/SIM) mode. The mass fragments (m/z) selected for the qualification and quantification were 55, 83 and 98 for mesityl oxide, and 58, 85 and 100 for IS. Quantification was performed by internal standard curve method and the method has been fully validated. This GC-EI-MS method is simple (one-step sample preparation), rapid (chromatographic cycle within 10 min, plus a 10 min postrun), specific (no interference peak shown in blank solution), accurate (mean recoveries within 88.0–97.3%), precise (intra- and inter-day precision ≤2.9%), sensitive (LOD ≤ 0.5 μg g−1 and LOQ ≤ 1.8 μg g−1), and stable (reference standard solution stable over 24 h). This method was successfully applied to the screening of mesityl oxide in three batches of enalapril maleate bulk drug.