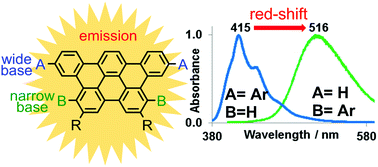
Nine new derivatives of the trapezoidal tribenzopentaphene (TBP) polycyclic aromatic hydrocarbon (PAH) were synthesized via the Suzuki–Miyaura palladium catalyzed cross-coupling reaction. The novel TBP derivatives, which bear various rigid and flexible aromatic groups either at their more accessible (R1) or congested (R2) bases, were fully characterized using high resolution mass spectrometry (HR-MS), nuclear magnetic resonance (NMR), UV-Vis absorption and emission spectroscopy. Our investigation reveals that extended conjugation between TBP and the aromatic side groups is possible when the latter are carefully selected and attached at the TBP wide base (R1), which causes an emission red-shift of the resulting target compounds. On the other hand, emission properties and density functional calculations suggest that attaching side groups at the sterically demanding base position (R2) induces a pronounced distortion from the planarity of the TBP central core structure.