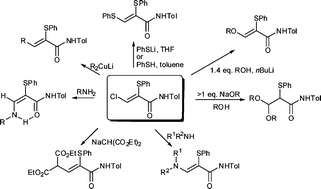
Synthetically versatile conjugate addition of a range of carbon, nitrogen, oxygen, sulfur and selenium nucleophiles to the highly functionalised 2-thio-3-chloroacrylamides is described. The stereochemical and synthetic features of this transformation are discussed in detail. In most instances, the nucleophile replaces the chloro substituent with retention of stereochemistry. With the oxygen nucleophiles, a second addition can occur leading to acetals, while with the nitrogen nucleophiles, E-Z isomerism occurs in the resulting enamine derivatives. The ratio of the E/Z isomers can be rationalised on the basis of the substituent and the level of oxidation.