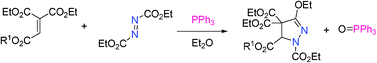Reaction of the Huisgen zwitterions, derived from triphenylphosphine and azodicarboxylates with activated alkenes has been studied. The reaction of various ethenetricarboxylates and diethyl azodicarboxylate with PPh3 gave pyrazolines efficiently. This pyrazoline synthesis is also applicable to 2-substituted acrylates such as trimethyl 2-phosphonoacrylate and methyl 2-(trifluoromethyl)acrylate. The possible reaction mechanism was discussed in comparison with the reaction of acetylenedicarboxylate and Huisgen zwitterions. The selective transformation of pyrazoline products have also been performed.