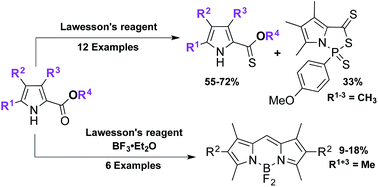
Reaction of 2-pyrrole carboxylates with Lawesson's reagent at elevated temperatures results in the corresponding thionoesters, concurrent with the production of a new class of pyrrole annulated with the (1,3,2)-thiazaphospholidine unit. X-ray crystallography was used to identify the pyrrolic thiazaphospholidine, which was found to have unique structural features compared to literature analogues. Addition of BF3·OEt2 to the thionation procedure was found to produce the corresponding F-BODIPY, constituting a four-step reaction in one-pot. The scope and limitations of these reactions involving the promiscuous Lawesson's reagent are discussed herein.