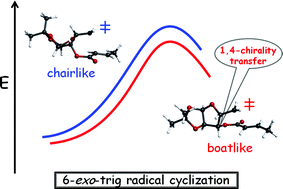
A computational investigation on the origin of the stereoselectivity of 6-exo-trig radical cyclization of α,β-unsaturated ester-tethered sugars has revealed that a boat-like transition state, which keeps the ester in a planar conformation, holds the chiral information. Following this model, the stereocenter to which the ester functionality is connected reports the chirality to the newly formed stereocenter via a 1,4-transfer mechanism.