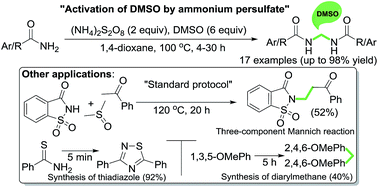
Activation of DMSO to work as an economical and environmentally benign one-carbon synthon has been achieved by using a bench-top reagent ammonium persulfate for general and efficient access to symmetrical methylenebisamides from primary amides. This methodology was used to achieve a three-component Mannich reaction using acetophenone, saccharin and DMSO to furnish a β-amino ketone. It also provided a metal-free synthesis of thiadiazole and bis(phenyl)methane. Effectively, this method uses DMSO as a safer surrogate to formaldehyde. A mechanism for methylenebisamide formation involving radical intermediates has been proposed based on mechanistic studies.