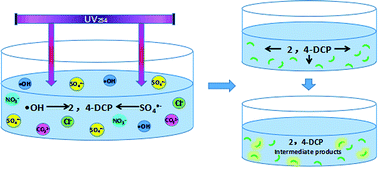
2,4-DCP is a high-toxicity phenol compound, which is difficult to remove, harmful to the health of people and seriously influences the aquatic ecosystems. In this study, the degradation of 2,4-DCP using UV/persulfate (UV/PS) process was investigated for the first time. The results showed pseudo-first-order rate constants of 2,4-DCP photo-degradation by UV/PS was 35.1 × 10−3 min−1. The reaction rate constants increased with pH increasing from 5 to 7 and then decreased at pH 8. Different anions (Cl−, HCO3− and NO3−) in water presented different effects on the photo-degradation reaction. The photo-degradation rates of 2,4-DCP in three actual water conditions (Xidong water works, Xijiu reservoir, Henshan reservoir) were higher than in the ultrapure water. Two possible (hydroxylated and dechlorinated) pathways for the degradation of 2,4-DCP by UV/PS were proposed. The luminescent bacteria inhibition rate greatly decreased with the concentration of 2,4-DCP decreasing in the reaction process.